Methane adsorption and dissociation on iron oxide oxygen carriers: the role of oxygen vacancies
Abstract
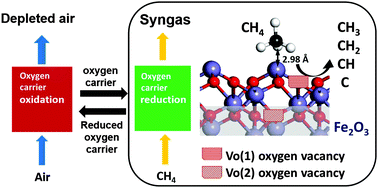
We performed ab initio DFT+U calculations to explore the interaction between methane and iron oxide oxygen carriers for chemical looping reaction systems. The adsorption of CH4 and CHx (x = 0–3) radicals on α-Fe2O3(001), and the influence of oxygen vacancies at the top surface and on the subsurface on the adsorption properties of the radicals was investigated. The adsorption strength for CH4 and C radicals at the top of the α-Fe2O3(001) surface in the presence of oxygen vacancies is lower than that on the stoichiometric surface. However, for methyl (CH3), methylene (CH2) and methine (CH) radicals, it is correspondingly higher. In contrast, the oxygen vacancy formation on the subsurface not only increases the adsorption strength of CH3, CH2 and CH radicals, but also facilitates C radical adsorption. We found that oxygen vacancies significantly affect the adsorption configuration of CHx radicals, and determine the probability of finding an adsorbed species in the stoichiometric region and the defective region at the surface. With the obtained adsorption geometries and energetics of these species adsorbed on the surface, we extend the analysis to CH4 dissociation under chemical looping reforming conditions. The distribution of adsorbed CH4 and CHx (x = 0–3) radicals is calculated and analyzed which reveals the relationship between adsorbed CHx radical configuration and oxygen vacancies in iron oxide. Also, the oxygen vacancies can significantly facilitate CH4 activation by lowering the dissociation barriers of CH3, CH2 and CH radicals. However, when the oxygen vacancy concentration reaches 2.67%, increasing the oxygen vacancy concentration cannot continue to lower the CH dissociation barrier. The study provides fundamental insights into the mechanism of CH4 dissociation on iron based oxygen carriers and also provide guidance to design more efficient oxygen carriers.

 Please wait while we load your content...
Please wait while we load your content...