The mechanism of the initial step of germanosilicate formation in solution: a first-principles molecular dynamics study†
Abstract
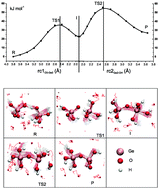
The condensation reactions between Ge(OH)4 and Si(OH)4 units in solution are studied to understand the mechanism and stable species during the initial steps of the formation process of Ge containing zeolites under basic conditions. The free energy of formation of (OH)3Ge–O–Ge–(OH)2O−, (OH)3Si–O–Si–(OH)2O−, (OH)3Ge–O–Si–(OH)2O− and (OH)3Si–O–Ge–(OH)2O− dimers is calculated with ab initio molecular dynamics and thermodynamic integration, including an explicit description of the water solvent molecules. Calculations show that the attack of the conjugated base (Ge(OH)3O− and Si(OH)3O−) proceeds with a smaller barrier at the Ge center. In addition, the formation of the pure germanate dimer is more favorable than that of the germano-silicate structure. These results explain the experimental observation of Ge–Ge and Si-Ge dimer species in solutions, with a few Si–Si ones.

 Please wait while we load your content...
Please wait while we load your content...