Effect of dolomite decomposition under CO2 on its multicycle CO2 capture behaviour under calcium looping conditions
Abstract
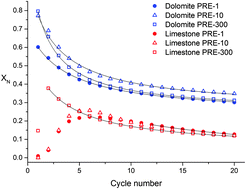
One of the major drawbacks that hinder the industrial competitiveness of the calcium looping (CaL) process for CO2 capture is the high temperature (∼930–950 °C) needed in practice to attain full calcination of limestone in a high CO2 partial pressure environment for short residence times as required. In this work, the multicycle CO2 capture performance of dolomite and limestone is analysed under realistic CaL conditions and using a reduced calcination temperature of 900 °C, which would serve to mitigate the energy penalty caused by integration of the CaL process into fossil fuel fired power plants. The results show that the fundamental mechanism of dolomite decomposition under CO2 has a major influence on its superior performance compared to limestone. The inert MgO grains resulting from dolomite decomposition help preserve a nanocrystalline CaO structure wherein carbonation in the solid-state diffusion controlled phase is promoted. The major role played by the dolomite decomposition mechanism under CO2 is clearly demonstrated by the multicycle CaO conversion behaviour observed for samples decomposed at different preheating rates. Limestone decomposition at slow heating rates yields a highly crystalline and poorly reactive CaCO3 structure that requires long periods to fully decarbonate and shows a severely reduced capture capacity in subsequent cycles. On the other hand, the nascent CaCO3 produced after dolomite half-decomposition consists of nanosized crystals with a fast decarbonation kinetics regardless of the preheating rate, thus fully decomposing from the very first cycle at a reduced calcination temperature into a CaO skeleton with enhanced reactivity as compared to limestone derived CaO.

 Please wait while we load your content...
Please wait while we load your content...