Solid-state NMR and DFT predictions of differences in COOH hydrogen bonding in odd and even numbered n-alkyl fatty acids†
Abstract
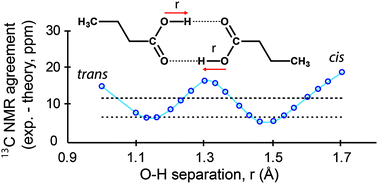
For nearly 140 years n-alkyl monocarboxylic acids have been known to exhibit unusual non-monotonic melting between odd and even numbered acids. This behavior has been rationalized in terms of packing density at the hydrocarbon tails, with COOH hydrogen bonding considered to be invariant among different acids. A recent ambiguity involving the COOH conformation between two crystal structures of lauric acid suggests that COOH structure and hydrogen bonding may play a role in these differences. Here, the two conflicting lauric acid crystal structures are further refined using lattice-including DFT refinement methods. Solid-state NMR (SSNMR) 13C chemical shift tensor data are employed to monitor refinement quality by comparing experimental and computed tensors. This comparison provides a more sensitive measure of structure than X-ray data due to SSNMR's ability to accurately locate hydrogens. Neither diffraction structure agrees with SSNMR data and an alternative is proposed involving a hydrogen disordered COOH moiety. The disordered hydrogen dynamically samples two most probable positions on the NMR timescale with O–H bond lengths of 1.16 and 1.46 Å. This disordered structure is consistent with SSNMR, IR and X-ray C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths. The hydrogen disorder appears to be restricted to even numbered acids based on undecanoic acid's 13COOH tensor data and C–O and C
O bond lengths. The hydrogen disorder appears to be restricted to even numbered acids based on undecanoic acid's 13COOH tensor data and C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths for other n-alkyl acids. This disorder in even numbered acids results in stronger hydrogen bonds than are found in odd acids and invites a reevaluation of the melting behavior of n-alkyl acids that includes these differences in hydrogen bonding.
O bond lengths for other n-alkyl acids. This disorder in even numbered acids results in stronger hydrogen bonds than are found in odd acids and invites a reevaluation of the melting behavior of n-alkyl acids that includes these differences in hydrogen bonding.

 Please wait while we load your content...
Please wait while we load your content...