Water distribution in a sorption enhanced methanation reactor by time resolved neutron imaging
Abstract
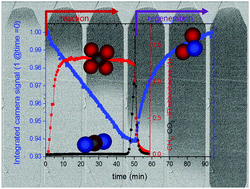
Water adsorption enhanced catalysis has been recently shown to greatly increase the conversion yield of CO2 methanation. However, the joint catalysis and adsorption process requires new reactor concepts. We measured the spatial water distribution in a model fixed bed reactor using time resolved neutron imaging. Due to the high neutron attenuation coefficient of hydrogen, the absorbed water in the sorption catalyst gives a high contrast allowing us to follow its formation and map its distribution. At the same time, the product gas was analysed by FTIR-gas analysis. The measurements provided crucial insights into the future design of sorption reactors: during the sorption enhanced reaction, a reaction front runs through the reactor. Once the extension of the reaction front reaches the exhaust, the conversion rate of sorption enhanced methanation decreases. The existence of a reaction front running through the reactor is prerequisite for a high conversion rate. We give a simple model of the experimental results, in particular the conditions, under which a reaction front is established. In particular the latter effect must be taken into account for the dimensions of a large scale reactor.
- This article is part of the themed collection: Neutron Scattering in Catalysis and Energy Materials

 Please wait while we load your content...
Please wait while we load your content...