Pyridine derivative/graphene nanoribbon composites as molecularly tunable heterogeneous electrocatalysts for the oxygen reduction reaction†
Abstract
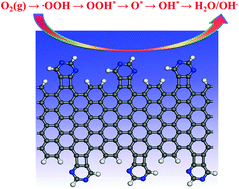
In this study, a strategy to design a new class of metal-free electrocatalysts for the oxygen reduction reaction (ORR) was proposed by means of density functional theory (DFT) computations. The electrocatalysts consist of various pyridine derivatives that are anchored on the edge sites of armchair graphene nanoribbons (AGNRs). Our results revealed that these anchored pyridine derivatives have considerably high stability, and the C atoms around the “external” N-dopant possess the largest positive charge, thus facilitating the ORR though a more efficient 4e pathway, in which the first electron is transferred into O2 molecules over a long range in the outer Helmholtz plane (i.e., the ET-OHP mechanism). Among these designed catalysts, the pyrimidine/AGNR exhibits the highest catalytic activity, which can be comparable to that of Pt-based catalysts. Therefore, our computations suggested that the combination of pyridine derivatives with graphene nanoribbons can constitute a novel and well-defined heterogeneous electrocatalyst with good stability and tunable active sites for the ORR, which provides a useful guidance to develop the next-generation of low-cost and metal-free electrocatalysts with accurate N species and content for the ORR in fuel cells.

 Please wait while we load your content...
Please wait while we load your content...