The structures and properties of proton- and alkali-bound cysteine dimers†
Abstract
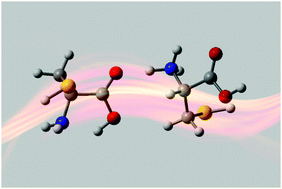
The proton-, lithium-, and sodium-bound cysteine dimers have been investigated in a joint computational and experimental infrared multiple photon dissociation (IRMPD) study. IRMPD spectra in the 1000–2000 cm−1 region show that protonation is localized on an amine group, and that intermolecular hydrogen bonding occurs between the protonated amine and the carbonyl oxygen of the neutral Cys moiety. Alkali-bound dimers adopt structures reminiscent of those observed for the monomeric Cys·Li+ and Cys·Na+ species. Calculations of the heavier Cys2·M+ (M = K, Rb or Cs) species suggest that these are significantly less strongly bound than the lighter (M = H, Li, or Na) dimers.

 Please wait while we load your content...
Please wait while we load your content...