Reactive oxygen species formed in organic lithium–oxygen batteries†
Abstract
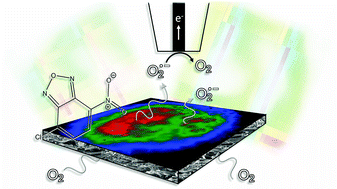
Li–oxygen batteries with organic electrolytes are of general interest because of their theoretically high gravimetric energy density. Among the great challenges for this storage technology is the generation of reactive oxygen species such as superoxides and peroxides that may react with the organic solvent molecules and other cell components. The generation of such species has been assumed to occur during the charging reaction. Here we show that superoxide is formed also during the discharge reaction in lithium ion-containing dimethyl sulfoxide electrolytes and is released into the solution. This is shown independently by fluorescence microscopy after reaction with the selective reagent 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole and by local detection using a microelectrode of a scanning electrochemcial microscope positioned in a defined distance of 10 to 90 μm above the gas diffusion electrode.
- This article is part of the themed collection: Bunsentagung 2016: Basic Mechanisms in Energy Conversion


 Please wait while we load your content...
Please wait while we load your content...