CHARMM force field parameterization protocol for self-assembling peptide amphiphiles: the Fmoc moiety†
Abstract
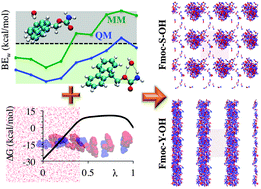
Aromatic peptide amphiphiles are known to self-assemble into nanostructures but the molecular level structure and the mechanism of formation of these nanostructures is not yet understood in detail. Molecular dynamic simulations using the CHARMM force field have been applied to a wide variety of peptide-based systems to obtain molecular level details of processes that are inaccessible with experimental techniques. However, this force field does not include parameters for the aromatic moieties which dictate the self-assembly of these systems. The standard CHARMM force field parameterization protocol uses hydrophilic interactions for the non-bonding parameters evaluation. However, to effectively reproduce the self-assembling behaviour of these molecules, the balance between the hydrophilic and hydrophobic nature of the molecule is essential. In this work, a modified parameterization protocol for the CHARMM force field for these aromatic moieties is presented. This protocol is applied for the specific case of the Fmoc moiety. The resulting set of parameters satisfies the conformational and interactions analysis and is able to reproduce experimental results such as the Fmoc–S–OMe water/octanol partition free energy and the self-assembly of Fmoc–S–OH and Fmoc–Y–OH into spherical micelles and fibres, respectively, while also providing detailed information on the mechanism of these processes. The effectiveness of the parameters for the Fmoc moiety validates the protocol as a robust approach to paramterise this class of compounds.

 Please wait while we load your content...
Please wait while we load your content...