Chirality-dependent structuration of protonated or sodiated polyphenylalanines: IRMPD and ion mobility studies†
Abstract
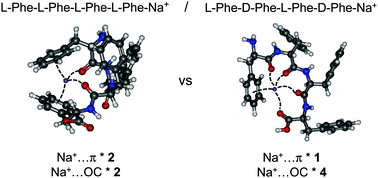
Ion mobility experiments are combined with Infra-Red Multiple Photon Dissociation (IRMPD) spectroscopy and quantum chemical calculations for assessing the role of chirality in the structure of protonated and sodiated di- or tetra-peptides. Sodiated systems show a strong chirality dependence of the competition between Na+⋯O and Na+⋯π interactions. Chirality effects are more subtle in protonated systems and manifest themselves by differences in the secondary interactions such hydrogen bonds between neutral groups or those involving the aromatic rings.

 Please wait while we load your content...
Please wait while we load your content...