1,1,2,2-Tetracyanocyclopropane (TCCP) as supramolecular synthon†
Abstract
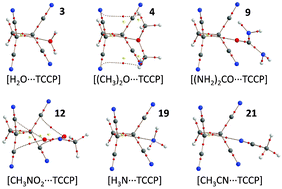
The 1,1,2,2-tetracyanocyclopropane (TCCP) unit presents a synthetically accessible and versatile synthon that can interact with lone-pair or π-electrons by ‘non-covalent carbon bonding’. Complexes of TCCP with common small molecules, anions, aromatics like fullerenes, amino acids and nucleobases were computed at the DFT BP86-D3/def2-TZVP level of theory. Binding energies vary between about −10 kcal mol−1 for neutral guests and −15 to −50 kcal mol−1 for anionic species. This is comparable to strong and very strong hydrogen bonding respectively. Thus, in addition to synthons that contain polarized hydrogen or halogen atoms, TCCP presents a new supramolecular synthon that awaits experimental exploitation.


 Please wait while we load your content...
Please wait while we load your content...