The role of conserved Cys residues in Brassica rapa auxin amidohydrolase: Cys139 is crucial for the enzyme activity and Cys320 regulates enzyme stability†
Abstract
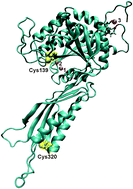
Brassica rapa auxin amidohydrolase (BrILL2) participates in the homeostasis of the plant hormones auxins by hydrolyzing the amino acid conjugates of auxins, thereby releasing the free active form of hormones. Herein, the potential role of the two conserved Cys residues of BrILL2 (at sequence positions 139 and 320) has been investigated by using interdisciplinary approaches and methods of molecular biology, biochemistry, biophysics and molecular modelling. The obtained results show that both Cys residues participate in the regulation of enzyme activity. Cys320 located in the satellite domain of the enzyme is mainly responsible for protein stability and regulation of enzyme activity through polymer formation, as has been revealed by enzyme kinetics and differential scanning calorimetry analysis of the BrILL2 wild type and mutants C320S and C139S. Cys139 positioned in the active site of the catalytic domain is involved in the coordination of one Mn2+ ion of the bimetal center and is crucial for the enzymatic activity. Although the point mutation Cys139 to Ser causes the loss of enzyme activity, it does not affect the metal binding to the BrILL2 enzyme, as has been shown by isothermal titration calorimetry, circular dichroism spectropolarimetry and differential scanning calorimetry data. MD simulations (200 ns) revealed a different active site architecture of the BrILL2C139S mutant in comparison to the wild type enzyme. Additional possible reasons for the inactivity of the BrILL2C139S mutant have been discussed based on MD simulations and MM-PBSA free energy calculations of BrILL2 enzyme complexes (wt and C139S mutant) with IPA-Ala as a substrate.

 Please wait while we load your content...
Please wait while we load your content...