On the nature of the solvated electron in ice Ih†
Abstract
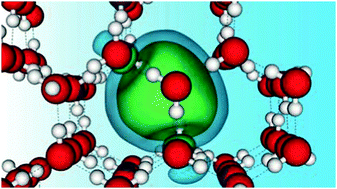
The water-solvated excess electron (EE) is a key chemical agent whose hallmark signature, its asymmetric optical absorption spectrum, continues to be a topic of debate. While nearly all investigation has focused on the liquid–water solvent, the fact that the crystalline-water solvated EE shows a very similar visible absorption pattern has remained largely unexplored. Here, we present spin-polarized density-functional theory calculations subject to periodic boundary conditions of the interplay between an EE and a number of intrinsic lattice defects in ice Ih. Our results show that the optical absorption signatures in the presence of three unsaturated hydrogen bonds (HB) are very similar to those observed experimentally. Its low-energy side can be attributed to transitions between the EE ground state and a single localized excited level, in a picture that is different from that for the liquid solvent, where this portion has been associated with hydrogen-like s → p excitations. The blue tail, on the other hand, relates to transitions between the EE ground state and delocalized excited states, which is in line with the bound-to-continuum transition interpretations for the EE in liquid water. Finally, we find that, depending on the number of dangling HBs participating in the EE trap, its charge density may spontaneously break the spin degeneracy through exchange interactions with the surrounding electrons, displaying the many-electron quantum nature of the EE problem in ice Ih.


 Please wait while we load your content...
Please wait while we load your content...