A computational study of lithium interaction with tetracyanoethylene (TCNE) and tetracyaniquinodimethane (TCNQ) molecules†
Abstract
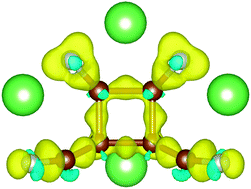
Tetracyanide molecules such as tetracyanoethylene (TCNE) and tetracyaniquinodimethane (TCNQ) have been proposed as promising candidate materials for organic battery electrodes, including lithium ion as well as sodium ion batteries. Their high theoretical capacities are in particular due to the possibility to store more than one alkali atom per molecule. Here, we present a density functional theory study of lithium attachment to TCNE and TCNQ. The trends in the Li binding strengths (which determines the electrode voltage) are presented between TCNE and TCNQ and as a function of the number of attached Li atoms. We show that multiple Li attachment induces non-trivial changes in the electronic structure. Electron donation from Li/Na is possible to higher (than LUMO) unoccupied molecular orbitals as well as Li-centered orbitals. Strain effects induced by Li attachment lead to significant changes in the electronic structure and can induce changes in orbital ordering. A cyclic structure stabilized by Li attachment to TCNE is identified. We conclude that the design of organic electrode materials should consider the energies of higher (than LUMO) orbitals as well as the effects of structural changes on the electronic structure.

 Please wait while we load your content...
Please wait while we load your content...