Simulations reveal the role of composition into the atomic-level flexibility of bioactive glass cements†
Abstract
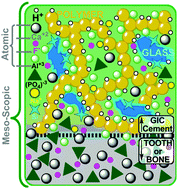
Bioactive glass ionomer cements (GICs), the reaction product of a fluoro–alumino–silicate glass and polyacrylic acid, have been in effective use in dentistry for over 40 years and more recently in orthopaedics and medical implantation. Their desirable properties have affirmed GIC's place in the medical materials community, yet are limited to non-load bearing applications due to the brittle nature of the hardened composite cement, thought to arise from the glass component and the interfaces it forms. Towards helping resolve the fundamental bases of the mechanical shortcomings of GICs, we report the 1st ever computational models of a GIC-relevant component. Ab initio molecular dynamics simulations were employed to generate and characterise three fluoro–alumino–silicate glasses of differing compositions with focus on resolving the atomic scale structural and dynamic contributions of aluminium, phosphorous and fluorine. Analyses of the glasses revealed rising F-content leading to the expansion of the glass network, compression of Al–F bonding, angular constraint at Al-pivots, localisation of alumino–phosphates and increased fluorine diffusion. Together, these changes to the structure, speciation and dynamics with raised fluorine content impart an overall rigidifying effect on the glass network, and suggest a predisposition to atomic-level inflexibility, which could manifest in the ionomer cements they form.

 Please wait while we load your content...
Please wait while we load your content...