Understanding the electromagnetic interaction of metal organic framework reactants in aqueous solution at microwave frequencies†
Abstract
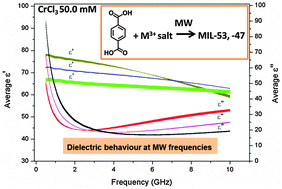
Preparation of metal organic frameworks (MOFs) via microwave heating is becoming increasingly popular due to reduced reaction times and enhanced control of MOF particle size. However, there is little understanding about the detailed interaction of the electric field portion of the wave with reactants during the synthesis of MOFs. In order to overcome this lack of fundamental understanding, information about the dielectric properties of the reactants is required. In this work the dielectric constants (ε′) and loss factors (ε′′) of benzene-1,4-dicarboxylic acid (H2BDC; also known as terephthalic acid) and a number of M(III) (M = metal) salts dissolved in deionized water were measured as a function of frequency, temperature and concentration and with varying anions and cations. Dielectric data confirm the aqueous M(III) salts to be strong microwave absorbers, particularly at 915 MHz. M(III) salts with mono-anionic ligands (for example chlorides and nitrates) exhibit higher losses than di-anionic salts (sulfates) demonstrating that the former are heated more effectively in an applied microwave field. Of the M(III) salts containing either singly- or doubly-charged anions, those containing Fe(III) have the highest loss indicating that they will heat more efficiently than other M(III) salts such as Cr(III) and Al(III). Interestingly, H2BDC exhibits little interaction with the electric field at microwave frequencies.


 Please wait while we load your content...
Please wait while we load your content...