Coupled optical absorption, charge carrier separation, and surface electrochemistry in surface disordered/hydrogenated TiO2 for enhanced PEC water splitting reaction†
Abstract
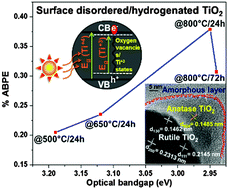
The central governing factors that influence the efficiency of photoelectrochemical (PEC) water splitting reaction are photon absorption, effective charge-carrier separation, and surface electrochemistry. Attempts to improve one of the three factors may debilitate other factors and we explore such issues in hydrogenated TiO2, wherein a significant increase in optical absorption has not resulted in a significant increase in PEC performance, which we attribute to the enhanced recombination rate due to the formation of amorphization/disorderness in the bulk during the hydrogenation process. To this end, we report a methodology to increase the charge-carrier separation with enhanced optical absorption of hydrogenated TiO2. Current methodology involves hydrogenation of non-metal (N and S) doped TiO2 which comprises (1) lowering of the band gap through shifting of the valence band via less electronegative non-metal N, S-doping, (2) lowering of the conduction band level and the band gap via formation of the Ti3+ state and oxygen vacancies by hydrogenation, and (3) material processing to obtain a disordered surface structure which favors higher electrocatalytic (EC) activity. This design strategy yields enhanced PEC activity (%ABPE = 0.38) for the N–S co-doped TiO2 sample hydrogenated at 800 °C for 24 h over possible combinations of N–S co-doped TiO2 samples hydrogenated at 500 °C/24 h, 650 °C/24 h and 800 °C/72 h. This suggests that hydrogenation at lower temperatures does not result in much increase in optical absorption and prolonged hydrogenation results in an increase in optical absorption but a decrease in charge carrier separation by forming disorderness/oxygen vacancies in the bulk. Furthermore, the difference in double layer capacitance (Cdl) calculated from electrochemical impedance spectroscopy (EIS) measurements of these samples reflects the change in the electrochemical surface area (ECSA) and facilitates assessing the key role of surface electrochemistry in PEC water splitting reaction. Additionally, we observed a blue-shift of the absorption spectrum and a decrease in both electrochemical (EC) and photoelectrochemical (PEC) activities after the removal of surface layers through focused ion beam (FIB) sputtering suggesting the importance of surface defects and photon absorption.

 Please wait while we load your content...
Please wait while we load your content...