An EPR study of ampullosporin A, a medium-length peptaibiotic, in bicelles and vesicles†
Abstract
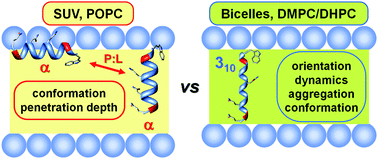
Ampullosporin A is a medium-length (14-amino acid long) hydrophobic peptide of the peptaibol family. In this work, electron paramagnetic resonance and circular dichroism spectroscopies were applied to study the interaction of synthetic ampullosporin A and three spin-labeled analogs with small unilamellar vesicles and bicelles. Zwitterionic vesicles were used to investigate the conformation and the penetration depth of the peptide at room temperature. Bicelles were employed in combination with EPR spectroscopy to study the order, dynamics, orientation, aggregation and the 3D-structure of the peptide at near physiological temperature. In the membrane, the peptide adopts a helical structure that changes in nature depending on the thickness of the membrane-mimetic system, from mostly α-helical in vesicles to a more elongated helix in bicelles, suggesting an increase in the 310-helical content. The orientation assumed by the peptide also shows a dependence on the membrane-mimetic system: in bicelles, ampullosporin A has a transmembrane orientation at a peptide-to-lipid (P : L) ratio of 1 : 100 and higher, while in vesicles it undergoes a transition from a parallel to a transmembrane orientation as a function of the P : L ratio. In bicelles, the peptide was found to be monomeric at a P : L ratio of 1 : 25 and lower. Overall, the comparison of the results obtained in the two membrane-mimetic systems showed that ampullosporin A has a rather flexible structure that readily adapts to the bilayer thickness.

 Please wait while we load your content...
Please wait while we load your content...