The role of unconventional stacking interactions in the supramolecular assemblies of Hg(ii) coordination compounds†
Abstract
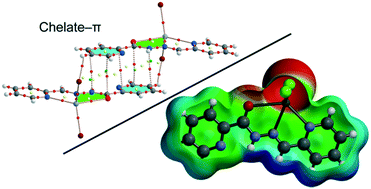
In this study, nine mercury(II) complexes of the composition [Hg(Ln)(X)2] (X = Cl, Br and I, n = 1–3), (L1 = 2-pyridine piconyl hydrazone); L2 = (2-acetylpyridine piconyl hydrazone) and L3 = (2-phenylpyridine piconyl hydrazone) are synthesized and spectroscopically characterized. Single-crystal X-ray crystallography showed that the molecular complexes can aggregate into larger entities depending upon the anion coordinated to the metal centre. Moreover, Hirshfeld surface (HS) analyses were employed to gain additional insight into interactions responsible for the packing of complexes 1–9. Quantitative examination of 2D fingerprint plots revealed, among others, the dominating participation of H⋯H and H⋯X interactions in the molecular packing. Moreover, C–H⋯X hydrogen bonds, π–π, and chelate-ring–π interactions are described and analysed by means of density functional theory (DFT) calculations since they play an important role in the construction of three-dimensional supramolecular frameworks. The influence of the halide on the energetic features of the assemblies has been also studied.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...