Stacking interactions between hydrogen-bridged and aromatic rings: study of crystal structures and quantum chemical calculations†
Abstract
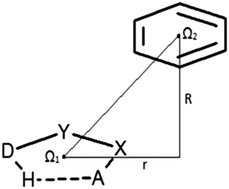
Geometric analysis of data from Cambridge Structural Database (CSD) reveals that contacts between planar hydrogen-bridged rings and C6-aromatic rings are mostly parallel stacked geometries. High-level quantum chemical calculations show that their interaction energies are comparable with interactions between two hydrogen-bridged rings. Namely, the interaction energy, at the CCSD(T)/CBS level, of the most stable geometry is −4.38 kcal mol−1, which is comparable with that of interaction between two hydrogen-bridged rings (−4.89 kcal mol−1) and significantly stronger than that of stacking between two benzene rings (−2.73 kcal mol−1).

 Please wait while we load your content...
Please wait while we load your content...