Competitive growth of crystals in vanadium–chromium slag
Abstract
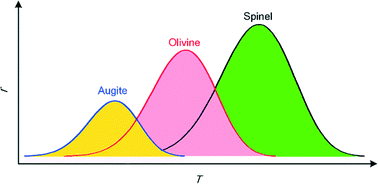
Based on the classic kinetic theory of crystallization, we have developed a mathematical model to describe the crystallization behavior of crystals in complex vanadium–chromium slag systems. The results show that the crystallization rates of spinels, olivines and augites are dominated by the component with the highest melting point. The sequence of the crystallization ability of crystals in vanadium–chromium slag is as follows: FeCr2O4 > Mg2SiO4 > FeV2O4 > MgSiO3 > Fe2TiO4 > Mn2SiO4 > MnSiO3 > Fe2SiO4. The optimum temperature ranges for the crystallization of these crystals are 1497–1547 K, 1380–1430 K, 1301–1351 K, 1192–1242 K, 1098–1148 K, 1069–1119 K, 1038–1088 K and 985–1035 K, respectively. We also find that with the increase in Cr2O3/V2O3 and decrease in SiO2/FeO, the total crystallization rate of spinels increases remarkably, while the TiO2 content has little effect on the crystallization of spinels in vanadium–chromium slag.

 Please wait while we load your content...
Please wait while we load your content...