Mild synthesis of {001} facet predominated Bi2O2CO3 clusters with outstanding simulated sunlight photocatalytic activities†
Abstract
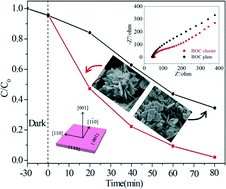
Bi2O2CO3 clusters made up of ultrathin nanosheets with predominated {001} facets were facilely synthesized via a template-free hydrothermal strategy at a mild temperature of 60 °C. Na2CO3 dosage and reaction temperature are confirmed to be key parameters to obtain the hydrolysis product of (Bi2O2)2+ from bismuth nitrate and provide a suitable microenvironment for the assembly of nanosheets. The sample exhibits obviously improved photocatalytic activity for the degradation and mineralization of Rhodamine B compared with thicker Bi2O2CO3 plates of less exposed {001} planes, with the reaction rate constant k enhanced by 3.4 fold. Photoluminescence and electrochemical impedance results confirm that the {001} facets are reactive and favorable for the separation and migration of photogenerated carriers, which primarily account for the superior photocatalytic behavior of the Bi2O2CO3 clusters. In addition, the enhanced specific surface area should also contribute to the improved photocatalytic activity. Based on the band edge positions of Bi2O2CO3 and the redox potentials of detected oxidative species, a possible migration mechanism of photogenerated e−/h+ pairs on the surface of Bi2O2CO3 is proposed. This work provides some new insight into the rational design and synthesis of facet-dependent semiconductor photocatalyst under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...