A thiadiazole-functionalized Zr(iv)-based metal–organic framework as a highly fluorescent probe for the selective detection of picric acid†
Abstract
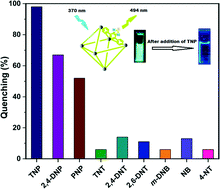
A new, strongly luminescent Zr(IV)-based metal–organic framework (MOF) material (1) having a UiO-68 (UiO = University of Oslo) framework topology and incorporating the π-conjugated, thiadiazole-functionalized H2BTDB {H2BTDB = 4,4′-(benzo[c][1,2,5]thiadiazole-4,7-diyl)dibenzoic acid} ligand was synthesized under solvothermal conditions (150 °C, 24 h) using ZrCl4 and the H2BTDB ligand in DMF (DMF = N,N-dimethylformamide). The phase-purity of as-synthesized 1 was ascertained by X-ray powder diffraction (XRPD) analysis, Fourier transform infrared (FT-IR) spectroscopy and elemental analysis. Based on the thermogravimetric analyses, 1 is thermally stable up to 400 °C. XRPD experiments verify that activated 1′ retains its crystallinity when exposed to water, acetic acid and 1 M HCl solutions. As revealed by the steady-state fluorescence titration experiments, the thermally activated form of the compound (1′) showed a selective sensing behaviour towards 2,4,6-trinitrophenol (TNP, commonly known as picric acid), even in the presence of other potentially competing nitroaromatic explosive compounds. The estimated detection limit of 1′ for sensing TNP in methanol suspension was found to be 1.63 × 10−6 M. The highest fluorescence quenching ability of TNP can be attributed to both energy and electron transfer processes as well as electrostatic interactions between the hydroxyl group of TNP and the Lewis basic N-donor sites of the BTDB ligand. Endowed with its excellent detection efficiency, 1′ is a promising luminescent sensor material for the long-term, practical sensing of TNP.

 Please wait while we load your content...
Please wait while we load your content...