Iodine salts of the pharmaceutical compound agomelatine: the effect of the symmetric H-bond on amide protonation†
Abstract
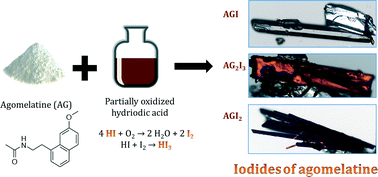
The search for new solid forms of an active pharmaceutical ingredient (API) is an important step in drug development. Often, an API has low water solubility, which then leads to low oral bioavailability. The problem can be solved by salt formation. One such API is agomelatine (AG), a melatonergic antidepressant. The aim of this work is to prepare iodide(s) of this compound. Three structurally different iodides of agomelatine were synthesized: agomelatine hydriodide trihydrate (AGI), agomelatine hemitriiodide (AG2I3) and agomelatine hemitriiodide iodine (AGI2). Their structures were solved from single-crystal X-ray diffraction data. In all of the structures, the agomelatine molecule was positively charged. Specifically, the amide oxygen was protonated, and in two of the structures (AG2I3 and AGI2), a symmetric hydrogen bond was formed. However, agomelatine is an amidic compound, and since amides are generally considered as neutral, in addition to SXRD, we present data from solid state NMR and the ΔpKa calculation to support the proton transfer and the salt formation.

 Please wait while we load your content...
Please wait while we load your content...