The structural effects of alkaline- and rare-earth element incorporation into thorium molybdates†
Abstract
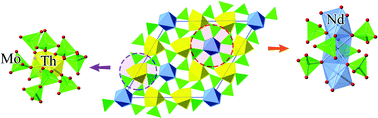
Four novel thorium molybdates containing alkaline-earth or rare-earth metals were isolated from high-temperature solid-state synthesis. The incorporation of divalent and trivalent cations into the thorium molybdate system results in complex structural topologies. A2MgTh3(MoO4)8 (A = K, Rb) which crystallizes in the space group C2/c is the first instance among the thorium molybdate family that incorporates alkaline-earth metals. Its crystal structure is based on complex channels composed of ThO8 square antiprisms and MoO4 tetrahedra arranged in a corner-sharing manner. K2SrTh2(MoO4)6, as the first thorium polymolybdate compound, is constructed from ThO8 square antiprisms and Mo4O16 tetramers. The Mo4O16 tetramers, lying in the (001) plane, are built from four edge-sharing MoO6 octahedra. Nd2Th3(MoO4)9 is the first thorium molybdate containing rare-earth cations. The resemblance of Nd2Th3(MoO4)9 with hexagonal-ThMo2O8 reveals its potential as a host for different trivalent transuranium elements. Raman spectra analysis shows that the different Mo polyhedral geometries (MoO4 tetrahedra and MoO6 octahedra) have significant effects on the vibrational bands of these compounds. The thermal behavior and stability of the newly obtained materials have been studied.

 Please wait while we load your content...
Please wait while we load your content...