Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site†
Abstract
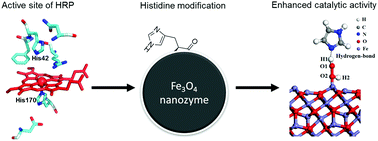
The Fe3O4 nanozyme was the first reported nanoparticle with intrinsic peroxidase-like activity and has been widely used in biomedicine. To optimize its catalytic activity, we introduced histidine residues onto the Fe3O4 nanoparticle surface in order to mimic the enzymatic microenvironment of natural peroxidase enzymes. Our results show that modification with a single amino acid could more than ten-fold improve the apparent affinity (KM) of the Fe3O4 nanozyme for the substrate H2O2 and enhanced its catalytic efficiency (kcat/KM) up to twenty fold. Thus we not only optimized the activity of the Fe3O4 nanozyme, but also provide a new rationale for improving the efficiency of nanomaterial-based catalysts by utilizing strategies observed in nature.

 Please wait while we load your content...
Please wait while we load your content...