Cascade photoredox/gold catalysis: access to multisubstituted indoles via aminoarylation of alkynes†
Abstract
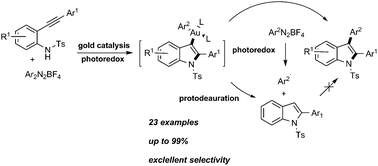
A new method for the synthesis of 3-arylindoles has been developed by visible light mediated dual gold/photoredox catalysis. This transformation has many features such as cascade catalysis, high efficiency, redox-neutral reaction conditions and good functional group tolerance. The reaction proceeds through the photoredox-promoted formation of an electrophilic arylgold(III) intermediate that undergoes coupling with the arylamine nucleophile.


 Please wait while we load your content...
Please wait while we load your content...