Metal-free C–H thioarylation of arenes using sulfoxides: a direct, general diaryl sulfide synthesis†
Abstract
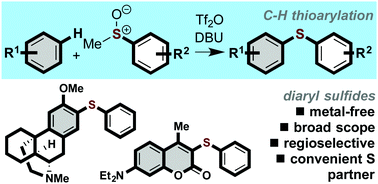
Metal-free C–H thioarylation of arenes and heteroarenes using methyl sulfoxides constitutes a general protocol for the synthesis of high value diaryl sulfides. The coupling of arenes and heteroarenes with in situ activated sulfoxides is regioselective, uses readily available starting materials, is operationally simple, and tolerates a wide range of functional groups.


 Please wait while we load your content...
Please wait while we load your content...