2-Nitropyrrole cross-coupling enables a second generation synthesis of the heronapyrrole antibiotic natural product family†
Abstract
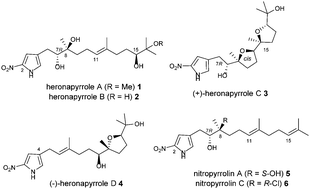
The heronapyrroles are a family of antibiotic natural products containing the rare 2-nitropyrrole motif. We report here a second generation total synthesis that circumvents problems of selective pyrrole nitration and alkene formation that limited the utility of previous approaches. Stille sp3–sp2 cross coupling of a novel 2-nitropyrrole fragment was developed to install the key C7,8 olefin with complete regiochemical control. An unusual Hunsdiecker-type decarboxylative halogenation was also identified, providing access to the complex vinyl stannane coupling partners required for formation of the natural product.


 Please wait while we load your content...
Please wait while we load your content...