A ‘photorelease, catch and photorelease’ strategy for bioconjugation utilizing a p-hydroxyphenacyl group†
Abstract
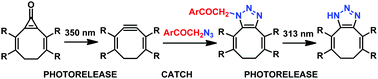
A bioorthogonal ‘catch and photorelease’ strategy, which combines alkyne–azide cycloaddition between p-hydroxyphenacyl azide and alkyne derivatives to form a 1,2,3-triazole adduct and subsequent photochemical release of the triazole moiety via a photo-Favorskii rearrangement, is introduced. The first step can also involve photorelease of a strained alkyne and its Cu-free click reaction with azide.


 Please wait while we load your content...
Please wait while we load your content...