A non-dissociative open-flask hydroboration with ammonia borane: ready synthesis of ammonia–trialkylboranes and aminodialkylboranes†
Abstract
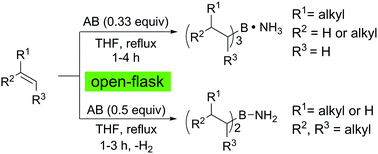
Under open-flask conditions, ammonia borane hydroborates olefins in refluxing tetrahydrofuran. Unlike conventional hydroboration, the Lewis base (ammonia) is not dissociated from the boron center. Terminal alkenes selectively provide ammonia–trialkylborane complexes. On the other hand, internal alkenes afford aminodialkylboranes via a metal-free hydroboration–dehydrogenation sequence. Alkaline hydrogen peroxide oxidation of the products provides the corresponding alcohols in high yields.

 Please wait while we load your content...
Please wait while we load your content...