Amine–boranes bearing borane-incompatible functionalities: application to selective amine protection and surface functionalization†
Abstract
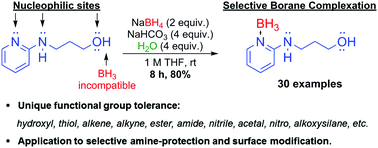
The first general open-flask synthesis of amine–boranes with inexpensive and readily available reagents, such as sodium borohydride, sodium bicarbonate, water, and the desired amines is described. Even amines bearing borane-reactive functionalities, such as alkene, alkyne, hydroxyl, thiol, ester, amide, nitrile, and nitro are well tolerated. Some of these novel amine–boranes represent stable molecules containing potentially incompatible electrophilic and nucleophilic centers in proximity. This convenient scalable synthesis provides a novel class of organic ligands for surface functionalization, as demonstrated by the formation of self-assembled layers of thiol- and alkoxysilane-bearing amine–boranes on gold and silica surfaces, respectively.


 Please wait while we load your content...
Please wait while we load your content...