A concise gram-scale synthesis of ht-13-A via a rhodium-catalyzed intramolecular C–H activation reaction†
Abstract
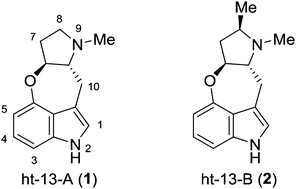
The enantioselective total synthesis of the 3,4-fused indole alkaloid ht-13-A has been achieved. The synthesis features a zinc-mediated propargylation, a rhodium-catalyzed intramolecular C–H activation reaction, and a methylamine-mediated cyclization. This concise and efficient synthetic approach can be applied for the gram-scale synthesis of ht-13-A.

 Please wait while we load your content...
Please wait while we load your content...