Mechanistic analysis of aliphatic β-lactones in Vibrio harveyi reveals a quorum sensing independent mode of action†
Abstract
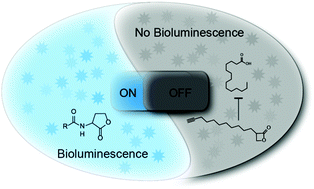
N-Acylhomoserine lactones are autoinducers of quorum sensing (QS) in Gram-negative bacteria. We exploit here the role of structurally related β-lactones in the inhibition of Vibrio harveyi bioluminescence and identify a derivative with nanomolar potency. Surprisingly, QS was not affected and combined proteomic/biochemical studies revealed insights into the cellular mode of action.

 Please wait while we load your content...
Please wait while we load your content...