MOF–aminoclay composites for superior CO2 capture, separation and enhanced catalytic activity in chemical fixation of CO2†
Abstract
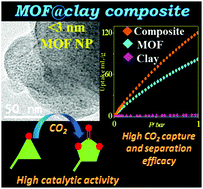
We delineate the growth and stabilization of ultra-small (2–3 nm) {Cu3(BTC)2(H2O)2·xH2O} MOF nanoparticles on a 2D layered aminoclay template. The composite shows significant CO2 uptake (5.35 mmol g−1 at 298 K, 1 bar; 46% higher than pristine bulk MOF), superior CO2 separation efficiency from CO2/N2 and CO2/CH4 mixtures and higher catalytic proficiency for chemical fixation of CO2 into cyclic carbonates.

 Please wait while we load your content...
Please wait while we load your content...