Diversity in a simple co-crystal: racemic and kryptoracemic behaviour†
Abstract
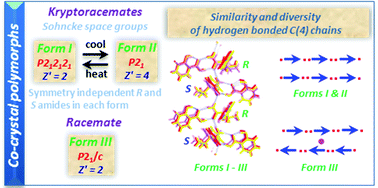
The crystal structure containing (±)-3-methyl-2-phenylbutyramide with salicylic acid is the first example of a kryptoracemate co-crystal. It exhibits the first temperature mediated reversible single-crystal to single-crystal transition between two kryptoracemate forms, in addition to crystallising in another, racemic, form. Theoretical calculations and structural analysis reveal that there are only small differences in both energy and packing arrangements between the three forms. These results suggest that co-crystals can be an opportunity to investigate kryptoracemate behaviour.


 Please wait while we load your content...
Please wait while we load your content...