Ligand-controlled stereodivergent 1,3-dipolar cycloaddition of azomethine ylides with 3-methyl-4-nitro-5-styrylisoxazoles†
Abstract
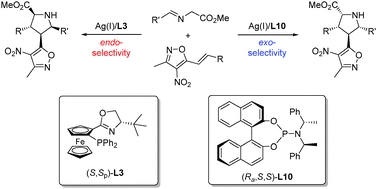
An unprecedented Ag(I)-catalyzed ligand-controlled stereodivergent 1,3-dipolar cycloaddition of azomethine ylides with 3-methyl-4-nitro-5-styrylisoxazoles has been developed to afford heterocycles bearing both methylisoxazole and pyrrolidine moieties. The endo- and exo-cycloadducts were obtained in good yields with excellent stereoselectivities, assisted by tBu-Phosferrox and phosphoramidite as chiral ligands, respectively.

 Please wait while we load your content...
Please wait while we load your content...