Gold(i) catalyzed tandem cyclization of propargylic esters to 4-acyloxy-1,2-dihydroquinolines†
Abstract
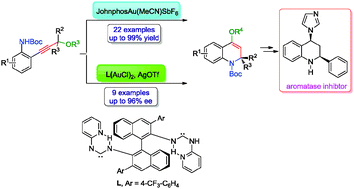
An effective synthetic protocol for structurally diverse 4-acyloxy-1,2-dihydroquinoline compounds has been accomplished by a gold(I)-catalyzed tandem [3,3]-rearrangement and intramolecular hydroamination of propargylic esters, affording the desired products in good yields. Moreover, the asymmetric variant of this cyclization has also been achieved using a chiral nitrogen acyclic carbene (NAC) gold(I) complex. These products have application in the enantioselective synthesis of an aromatase inhibitor within three simple steps.

 Please wait while we load your content...
Please wait while we load your content...