Mechanistic insights into light-driven graphene-induced peroxide decomposition: radical generation and disproportionation†
Abstract
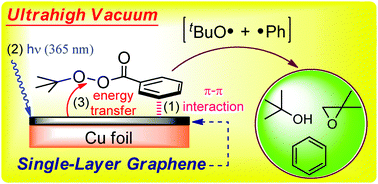
Interaction between adsorbed t-butyl peroxybenzoate and photoexcited graphene rendered trapped phenyl and t-butoxy radicals. Post-irradiation thermal desorption showed benzene, t-butanol, and isobutylene oxide as the end products. The required hydrogen atoms were obtained via the radical disproportionation. Graphene enabled radical species to be captured and their on-surface chemistry to be revealed.

 Please wait while we load your content...
Please wait while we load your content...