A palladium-catalyzed intramolecular carbonylative annulation reaction for the synthesis of 4,5-fused tricyclic 2-quinolones†
Abstract
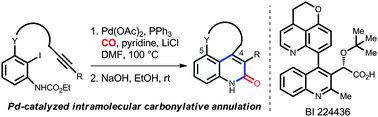
A concise and efficient synthetic route to 4,5-fused tricyclic 2-quinolones through the palladium-catalyzed carbonylative annulation of alkyne-tethered N-substituted o-iodoanilines has been developed. This reaction proceeds smoothly under mild reaction conditions and exhibits exceptional tolerance to a variety of functional groups. It has been successfully applied to the efficient synthesis of BI 224436, an HIV integrase inhibitor.

 Please wait while we load your content...
Please wait while we load your content...