Highly regioselective meta arylation of oxalyl amide-protected β-arylethylamine via the Catellani reaction†
Abstract
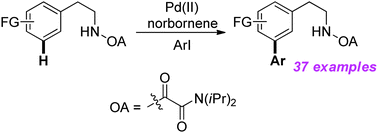
The first bidentate directing group assisted highly selective meta arylation of β-arylethylamine derivatives via palladium/norbornene catalysis is reported, and the range of aryl iodides for the oxalyl amide assisted meta-selective arylation reactions is broadest yet reported. This meta arylation also proceeds well with thiophene derivatives, giving the corresponding products in satisfactory yields. And three-step functionalization of arylethyloxalamide with three different functional groups is successfully performed.

 Please wait while we load your content...
Please wait while we load your content...