Regioselective construction of diverse and multifunctionalized 2-hydroxybenzophenones for sun protection by indium(iii)-catalyzed benzannulation†
Abstract
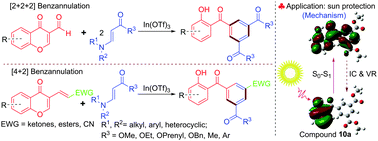
Diverse and functionalized 2-hydroxybenzophenone derivatives were synthesized efficiently in good to excellent yield via the highly regioselective indium(III)-catalyzed [2+2+2] benzannulation of 3-formylchromones with β-enamino esters or β-enamino ketones. This benzannulation reaction proceeds via a domino Michael/retro-Michael/6π-electrocyclization/deformylation reaction. In addition, 2-hydroxybenzophenones were also prepared by the indium(III)-catalyzed [4+2] benzannulation reaction between 3-substituted chromen-4-ones and β-enamino esters or β-enamino ketones. Furthermore, the effects of substituents and π conjugation on the characteristics of the UV-Vis spectrum of synthesized 2-hydroxybenzophenones were examined. Compound 10s exhibited higher sun protection activity than oxybenzone which is used in most popular sunscreens.


 Please wait while we load your content...
Please wait while we load your content...