Revealing instability and irreversibility in nonaqueous sodium–O2 battery chemistry†
Abstract
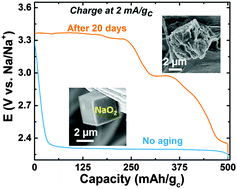
Charging kinetics and reversibility of Na–O2 batteries can be influenced greatly by the particle size of NaO2 formed upon discharge, and exposure time (reactivity) of NaO2 to the electrolyte. Micrometer-sized NaO2 cubes formed at high discharge rates were charged at smaller overpotentials compared to nanometer-sized counterparts formed at low rates.

 Please wait while we load your content...
Please wait while we load your content...