Oxygen insertion into boroles as a route to 1,2-oxaborines†
Abstract
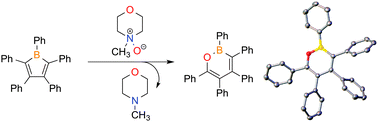
The synthesis of 1,2-oxaborines is accomplished via the reaction of pentaarylboroles with N-methylmorpholine-N-oxide via a 1,1-insertion reaction. The aromatic nature of 1,2-oxaborines was evaluated by computing nuclear independent chemical shift (NICS) values. Collectively, the experimental and computational studies indicate the unsaturated central BOC4 ring has appreciable aromatic character.

 Please wait while we load your content...
Please wait while we load your content...