Olefin hydrosilylation catalyzed by cationic nickel(ii) allyl complexes: a non-innocent allyl ligand-assisted mechanism†
Abstract
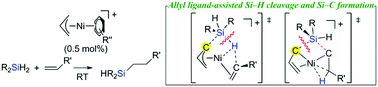
The arene-supported cationic nickel allyl complexes serve as good catalysts for olefin hydrosilylation at room temperature. Detailed mechanistic studies based on experiments and DFT calculations support the novel mechanism, which includes the facile Si–H bond cleavage and Si–C bond formation, assisted by a non-innocent allyl ligand.

 Please wait while we load your content...
Please wait while we load your content...