Acyl hydrolases from trans-AT polyketide synthases target acetyl units on acyl carrier proteins†
Abstract
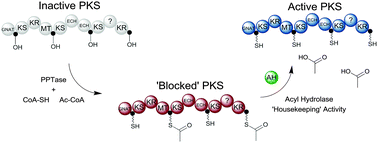
Acyl hydrolase (AH) domains are a common feature of trans-AT PKSs. They have been hypothesised to perform a proofreading function by removing acyl chains from stalled sites. This study determines the substrate tolerance of the AH PedC for a range of acyl-ACPs. Clear preference towards short, linear acyl-ACPs is shown, with acetyl-ACP the best substrate. These results imply a more targeted housekeeping role for PedC: namely the removal of unwanted acetyl groups from ACP domains caused by erroneous transfer of acetyl-CoA, or possibly by decarboxylation of malonyl-ACP.

 Please wait while we load your content...
Please wait while we load your content...