Tetrahedral cage complex with planar vertices: selective synthesis of Pt4L6 cage complexes involving hydrogen bonds driven by halide binding†
Abstract
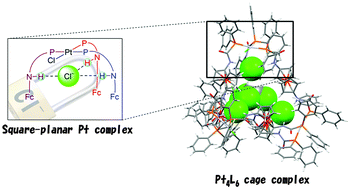
The reaction of Fe(η-C5H4NHC(O)PPh2)2 (L) with PtX2(PPh3)2 selectively formed cage-shaped complexes formulated as [(PtX)4(L)6]X4·CHCl3 (X = Cl, Br), in which the four square-planar Pt fragments were situated at each vertex and the six Ls were located in each side of a tetrahedral framework and hydrogen bonds existed between the NH groups in Ls and X− ions inside the cage.

 Please wait while we load your content...
Please wait while we load your content...