Toward the stereochemical assignment and synthesis of hemicalide: DP4f GIAO-NMR analysis and synthesis of a reassigned C16–C28 subunit†
Abstract
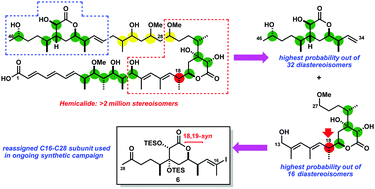
Using the DP4f GIAO-NMR method, the stereochemistry of hemicalide was computationally analysed, resulting in a reassignment at C18 as supported by improved NMR shift correlations with a model C13–C25 fragment 23. An advanced C16–C28 subunit 6 of this potent anticancer agent was then synthesised with the revised 18,19-syn relationship.

 Please wait while we load your content...
Please wait while we load your content...