In situ activation of a doxorubicin prodrug using imaging-capable nanoparticles†
Abstract
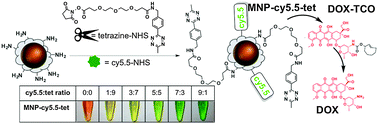
A general strategy for image-guided prodrug activation using fluorescently-labeled magnetic iron oxide nanoparticles is described. It is based on a recently developed concept in bio-orthogonal inverse-electron demand Diels–Alder chemistry, which is termed ‘click-to-release’. To illustrate a potential new biomedical application of the chemistry, the nanoparticles were modified with tetrazine, as well as near infrared fluorescent (NIRF) cy5.5 dye, while doxorubicin was converted into a prodrug. The nanoparticles taken up by the MDA-MB-231 breast cancer cells efficiently converted the prodrug of doxorubicin into the biologically active chemotherapeutic doxorubicin form.

 Please wait while we load your content...
Please wait while we load your content...