Synthesis of 2-fluoro-2-pyrrolines via tandem reaction of α-trifluoromethyl-α,β-unsaturated carbonyl compounds with N-tosylated 2-aminomalonates†
Abstract
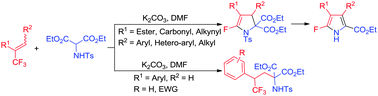
A simple base-mediated tandem SN2′/SNV reaction of the readily available α-trifluoromethyl-α,β-unsaturated carbonyl compounds with N-tosylated 2-aminomalonates was developed, which provide an efficient access to functionalized tetrasubstituted 2-fluoro-2-pyrrolines in good to excellent yields. In contrast, simple 1,2-nucleophilic adducts were produced when α-trifluoromethyl styrenes were used.

 Please wait while we load your content...
Please wait while we load your content...